Zebrafish
Zebrafish Radiobiology Lab
Profile
Shen Hongyuan
Email: snrsh@nus.edu.sg
Shen Hongyuan received her B.Sc. (Hons) in Life Sciences with concentration in Molecular and Cell Biology from National University of Singapore. After graduation, she joined Ding Xiang Liu’s lab in IMCB/A*STAR as a lab biologist working on genetic engineering of recombinant Coronaviruses as viral vectors harboring reporter genes for gene delivery. She received her PhD training in zebrafish development under the guidance of Vladimir Korzh in IMCB/A*STAR. During her PhD, she conducted Tol2-transposon mediated mutagenesis screen in zebrafish from which she isolated the silent K+ channel subunit mutant kcng4b and characterized its novel function in inflating the embryonic brain ventricle. As a postdoc, she generated the zebrafish ikk2 mutant and studied the function of Ikk2/NFkB signaling in early embryonic development. She joined SNRSI in 2016, and was attached to the Institute for Radiological Protection and Nuclear Safety (IRSN, France) where she investigated chromosomal aberration complexity produced by neutron fields in case of a critical nuclear accident. In 2019, she started her own lab utilizing zebrafish as a premier animal model for radiobiology research. The overall goal of her lab is to understand the mechanisms controlling the spatiotemporal dynamics of cells in their native tissue environmental niche when an intact biological organism is exposed to ionizing radiation, and hence derive potential intervention points whereby radiation-induced damage could be dampened.
Research
Research focus
Precise quantification of radiation damage in a biological entity in a timely manner is a major challenge in radiobiology. Bioassays are often carried out hours, days or even years after exposure, when the body has already elicited its intrinsic cellular repair mechanisms, rendering the assays only detecting results of recovery instead of the situation at the point of exposure. This is particularly true when quantifying low-dose ionizing radiation damage, as the amount of damage could be quickly reduced to minimum or basal level if the narrow time window is missed. Our group utilizes zebrafish to understand ionizing radiation damage towards biological organisms. By exploiting the optical transparency and genetic amenability of zebrafish embryos, we aim to understand the real-time spatiotemporal dynamics of molecular and cellular events happening when an intact organism is exposed to ionizing radiation.
Projects
- Accurate sensing and quantification of ionizing radiation damage in zebrafish
Zebrafish is widely recognized as a premier animal model in many aspects of biomedical research, its usage in ionizing radiation research is however still emerging. Currently, there is a lack of specific dosimetry method for accurate quantification of radiation damage. On the other hand, the small size (1.9-3.5 mm) and high mitotic index at embryonic stages allow chromosome aberrations to be microscopically visible through establishing high quality metaphase plates by direct blocking self-proliferating progenitor cells at mitosis. Our group is developing novel cytogenetic methods based on well-established dosimetry methods routinely applied to human lymphocytes. These newly established cytogenetic techniques aim to assess radiation damage in each individual zebrafish embryos, thus expanding the usage of zebrafish in environmental monitoring of radiosensitivity and radiation-induced genotoxicity studies of aquatic species.
- Developing transgenic zebrafish lines for real-time reporting of radiation damage
The most attractive feature of zebrafish lies in the optical transparency and genetic amenability of their embryos and larvae. These two traits make them ideal candidates for live imaging of cellular events after radiation exposure, revealing spatiotemporal changes in subcellular details under natural physiology. Our group is developing novel transgenic zebrafish lines that can serve as real-time reporters of radiation damage in vivo. Among them, Tg(ubi:secA5-mVenus, cryaa:mCherry)ns301 (abbreviated as secA5ns301), an apoptosis reporter line that expresses ANNEXIN V fused with mVenus proteins, enables visualizing radiation-induced cell death at single-cell resolution throughout the entire zebrafish. Using Confocal- and Lightsheet-based in vivo imaging approach, we are investigating tissue-specific radiosensitivity at the whole-organism level, including the dynamics of radiation-induced apoptosis in real-time using transgenic zebrafish.
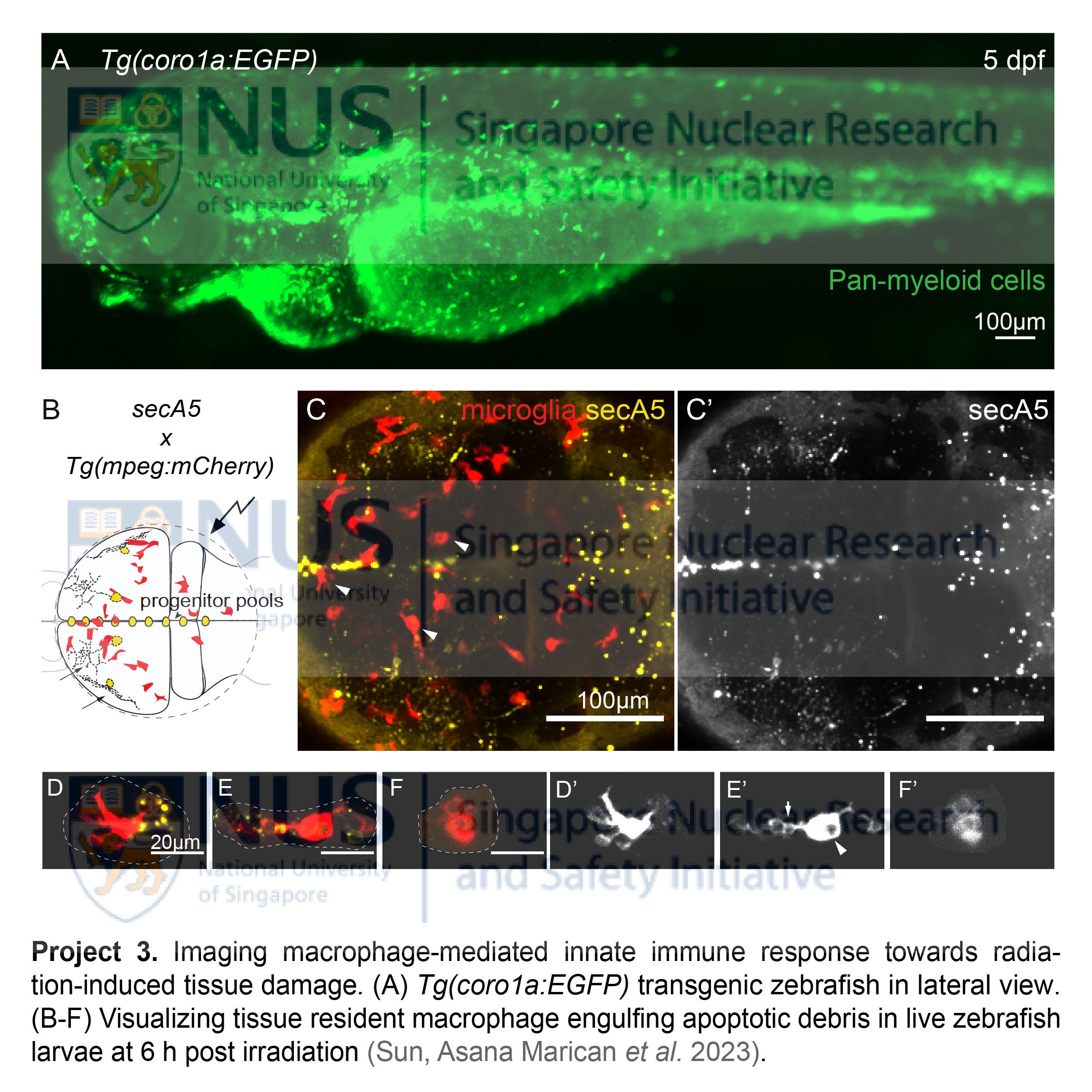
- Live imaging macrophage-mediated innate immune response towards radiation damage
Tissue resident macrophages, a major constituent of innate immunity, are the first few responders after radiation-induced tissue damage. These cells travel immediately to the injury sites to engulf apoptotic debris and elicit intricate repair functions essential to restore tissue homeostasis. In human patients exposed to radiation, myeloid cell numbers decline drastically within a short period, with their restoration tightly linked to patient survival rate. To date, the spatiotemporal dynamics of them remain elusive, as few systems could provide us a glimpse of these highly active cells in action within their native tissue environment. Exploiting the live imaging potential of the transparent zebrafish, our group is using transgenic lines, namely the pan-myeloid Tg(coro1a:EGFP)hkz04t and macrophage-specific Tg(mpeg:mCherry)gl23, to visualize the actual phagocytic events upon radiation exposure. Equipped with these powerful tools, we aim to dissect the distinct behaviors of tissue resident macrophages after radiation exposure, with focus on their phagocytosis, mobility and migrations. We aim to address how these innate immune cells 1) respond to radiation damage during the acute and latent phases; 2) engulf apoptotic debris and exert their repair functions; 3) revert back to their normal state to maintain tissue homeostasis or, if the previous attempts fail, 4) cause chronic inflammation and tissue injury (i.e. radiation-induced fibrosis) in the long term.
List of Publications
1. Beh LK, Shen H. Genotyping Zebrafish Point Mutant by Allele-Specific Blocking PCR. Zebrafish 2024; 21:297-299.
2. Asana Marican HT, Shen H. Dynamics of Chromosome Aberrations and Cell Death in Zebrafish Embryos Exposed to 137Cs Total-Body Irradiation. Environ Sci Technol 2024; 58:2204-2213.
3. Sun LWH, Asana Marican HT, Beh LK, Shen H. Imaging the radioprotective effect of amifostine in the developing brain using an apoptosis-reporting transgenic zebrafish. International Journal of Radiation Biology 2024; 100:433-444.
4. Sun LWH, Asana Marican HT, Shen H. In Vivo Imaging of Radiation-Induced Apoptosis at Single-Cell Resolution in Transgenic Zebrafish Embryos. Radiation Research 2023; 199:229-239.
5. Asana Marican HT, Shen H. Metaphase-Based Cytogenetic Approach Identifies Radiation-Induced Chromosome and Chromatid Aberrations in Zebrafish Embryos. Radiation Research 2022; 197:261-269.
6. Asana Marican HT, Sun LWH, Shen H. A Simple Method to Establish Metaphase Chromosomes from Individual Zebrafish Embryos. Zebrafish 2021; 18:338-341.
7. Tan YW, Fung TS, Shen H, Huang M, Liu DX. Coronavirus infectious bronchitis virus non-structural proteins 8 and 12 form stable complex independent of the non-translated regions of viral RNA and other viral proteins. Virology 2018; 513:75-84.
8. Shen H, Shin EM, Lee S, Mathavan S, Koh H, Osato M, et al. Ikk2 regulates cytokinesis during vertebrate development. Sci Rep 2017; 7:8094.
9. Shen H, Bocksteins E, Kondrychyn I, Snyders D, Korzh V. Functional antagonism of voltage-gated K+ channel alpha-subunits in the developing brain ventricular system. Development 2016; 143:4249-4260.
10. Teh C, Sun G, Shen H, Korzh V, Wohland T. Modulating the expression level of secreted Wnt3 influences cerebellum development in zebrafish transgenics. Development 2015; 142:3721-33.
11. Fang S, Shen H, Wang J, Tay FP, Liu DX. Functional and genetic studies of the substrate specificity of coronavirus infectious bronchitis virus 3C-like proteinase. Journal of virology 2010; 84:7325-36.
12. Shen H, Fang SG, Chen B, Chen G, Tay FP, Liu DX. Towards construction of viral vectors based on avian coronavirus infectious bronchitis virus for gene delivery and vaccine development. Journal of virological methods 2009; 160:48-56.
13. Fang SG, Shen H, Wang J, Tay FP, Liu DX. Proteolytic processing of polyproteins 1a and 1ab between non-structural proteins 10 and 11/12 of Coronavirus infectious bronchitis virus is dispensable for viral replication in cultured cells. Virology 2008; 379:175-80.