Bioindicators
Bioindicators of Radiation Exposure Lab
Research Team:
- Christelle Chua (PI, Senior Research Scientist)
- Chew Zi Huai (Research Assistant)
- Valerie Goh (Research Fellow)
- Lance Tay (Research Associate)
- Tan Han Wei (Research Associate)
- Teo Shu Xian (Research Assistant)
- Jonathan Yeo (Research Associate)
Our research
Elucidating mechanisms of radioadaptive effects and individual radiosensitivity
Preliminary research in our group has yielded a pool of biological indicators that change in response to chronic and/or low dose radiation exposure. These include genes involved in inflammation, tumour suppression and DNA repair. Interestingly, there are indications that radioadaptive effects can occur in situations of prior low dose exposure followed by a high challenge dose. This is evidenced by reduced levels of cell death and DNA damage markers. Moreover, it appears that the pathway choice of DNA repair may be altered with prior low dose radiation exposure. All these imply that low dose radiation exposure may alter the biological response. We thus seek to explore the mechanisms underlying these responses. Undergirding this research will be the acknowledgement that the Singapore population is genetically diverse and may be biologically different from other populations studied in literature. The research will thus seek also to identify biological indicators which may reflect differences in radiosensitivity.
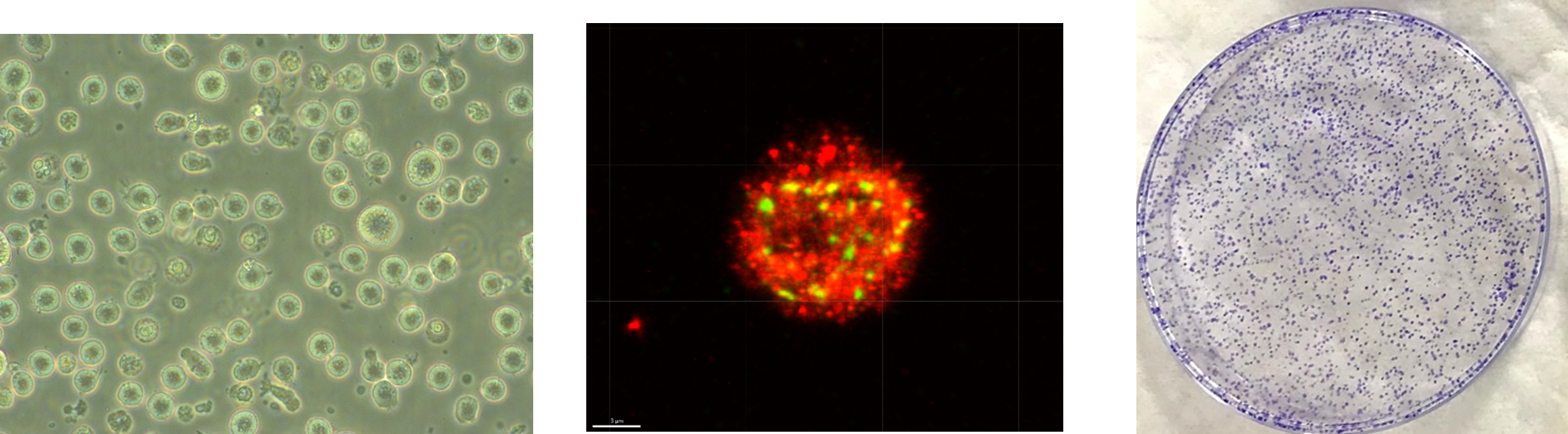
Fig 1. Cells employ a complex network of DNA repair pathways to respond to damage induced by radiation exposure. Differences in levels of DNA damage repair molecules and subsequent cell death or cell survival would suggest that cells may adopt different DNA damage responses depending on whether prior low doses of radiation exposure have occurred. Different biological responses may also occur in response to different radiation types. (A) Lymphoblastoid cells derived from human donors serve as a model to study radiobiological effects. (B) Labelling of cell for DNA damage foci (Green: ɣ-H2AX; Red: BRCA1). (C) The ability of individual cells to form colonies reflect their ability to survive and proliferate and exposure to various doses of radiation.
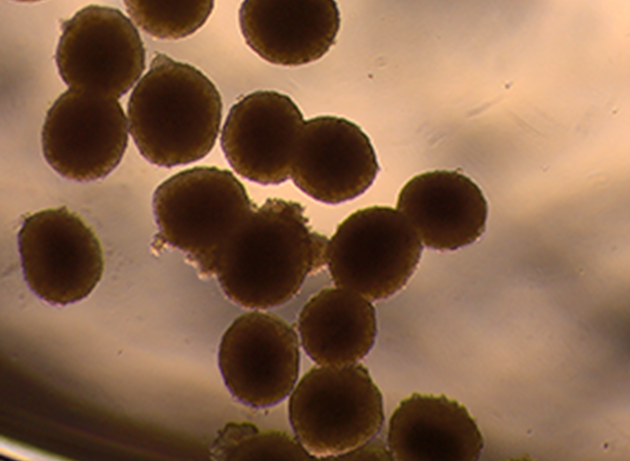

Fig 2. Spheroids cultured in microfluidics chips can serve as a model to study the effects of chronic low dose radiation exposure in a physiologically relevant cellular environment. (A) Liver spheroids. (B) Studies can be conducted on the impact of fate determination in hematopoietic stem cells in a bone marrow model, which is particularly susceptible to radiation exposure.
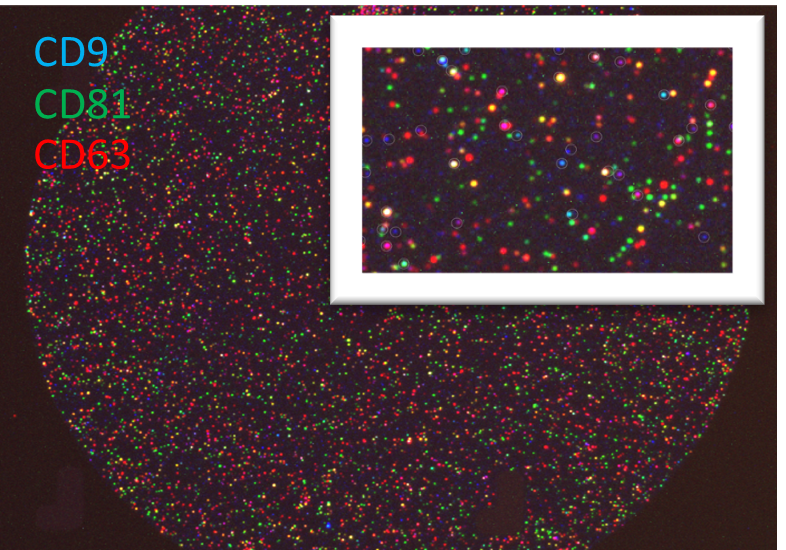
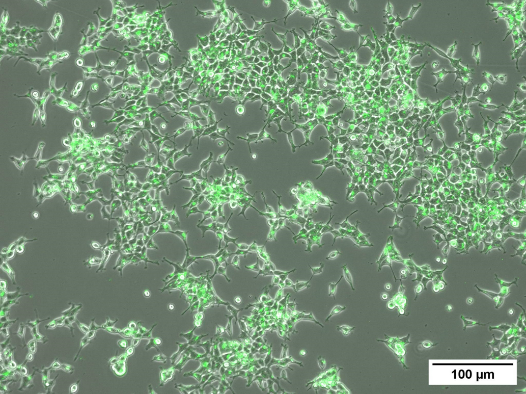
Fig 3. Intercellular signalling via extracellular vesicles (EVs) have been identified as one way in which biological response to radiation is mediated. Investigations into the type and amount of cargo carried by EVs released after radiation exposure from various cell types will give insight into the biological responses after different doses. (A) Labelling of tetraspanin markers on EVs isolated from plasma of irradiated blood samples. (B) GFP-labelled EVs in HEK cells.
Enhancement of protocols for dose assessment of radiation exposed individuals
Dose assessment of radiation-exposed individuals is largely conducted through cytogenetic-based dose response curves. With the capability to accurately perform biodosimetry using the dicentric assay, it is our goal to develop effective tools to enhance biodosimetry techniques, through methods such as reduction of hands-on operator time and dependency on commercial reagents and software. At the same time, it is necessary to ensure that accurate biodosimetry extends to cases of non-uniform and/or low dose exposure, and in a wide variety of differently-radiosensitive individuals. Samples from individuals exposed to radiation as part of medical or occupational procedures will serve as a pool on which analysis can be performed.
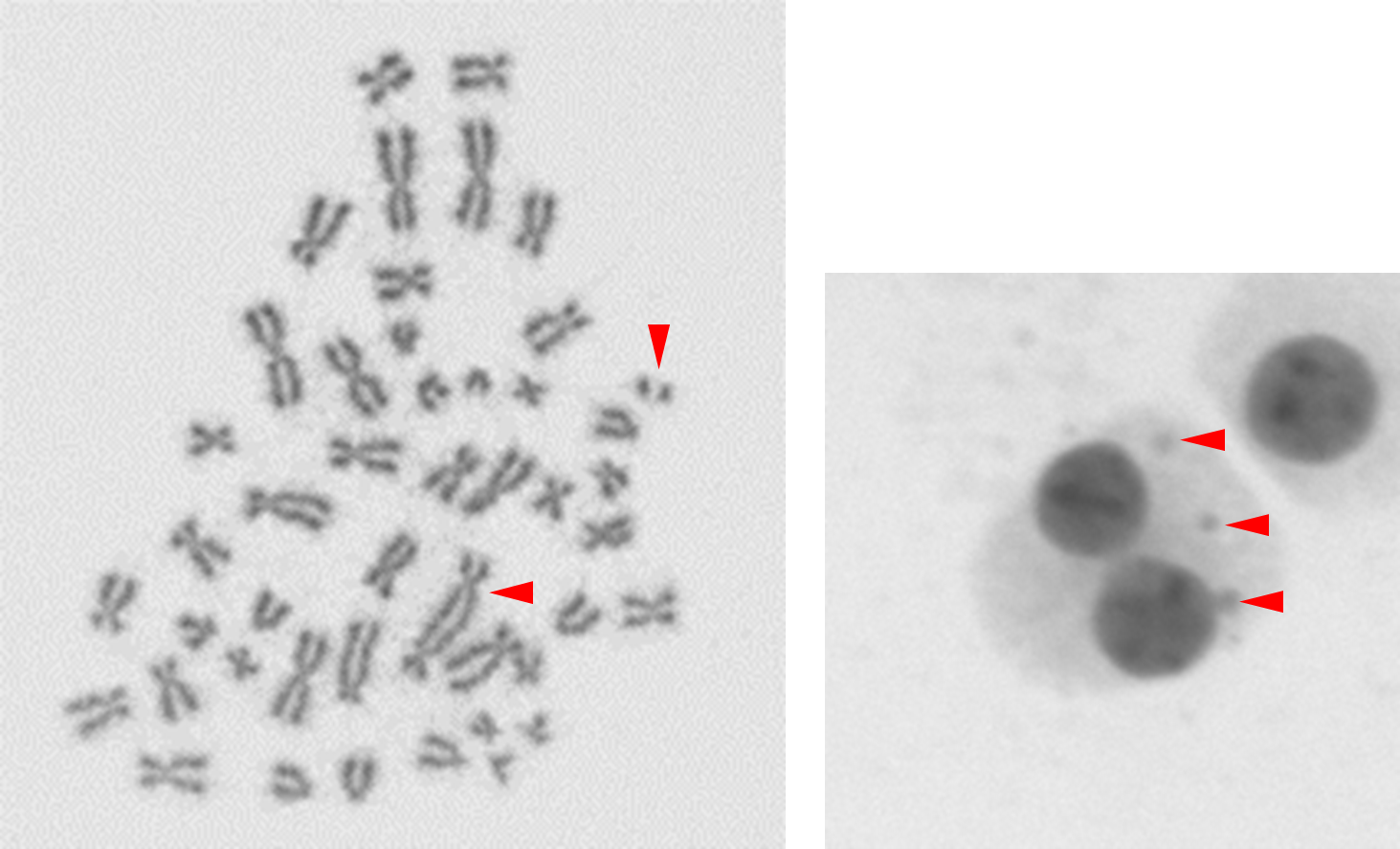
Fig 1. Examples of cytogenetic assay endpoints to determine the dose that a sample has received. (A) A dicentric chromosome with an accompanying acentric fragment in a metaphase spread. (B) 3 micronuclei in a binucleated cell.
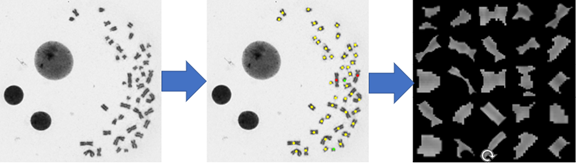
Fig 2. Automated dicentric detection using image detection and machine learning techniques will enhance the scalability, speed and accuracy of the assay.
Representative publications:
Yeo JJW, Goh VST, Teo SX, Chew ZH, Chua CEL. Automated dicentric scoring system in Singapore Nuclear Research and Safety Initiative. Ann ICRP. In press.
Tay LLT, Chua CEL. Hematopoietic stem cells in an organ-on-a-chip system for long-term ionising radiation studies. Ann ICRP. In press.
Dehkordi SR, Wong IT-L, Ni J, Luebeck J, Zhu K, Prasad G, Krockenberger L, Xu G, Chowdhury B, Rajkumar U, Caplin A, Muliaditan D, Coruh C, Jin Q, Turner K, Teo SX, Pang AWC, Alexandrov LB, Chua CEL, Furnari FB, Paulson TG, Law JA, Chang HY, Yue F, DasGupta R, Zhao J, Mischel PS and Bafna V. Breakage fusion bridge cycles drive high oncogene number with moderate intratumoural heterogeneity. Nat Commun 2025; 16:1497
Tan HW, Chua CEL. Radioadaptive responses induced by chronic low dose radiation in human lymphocytes. Proceedings of the sixth International Symposium on the System of Radiological Protection. Ann ICRP 2023; 52(S1).
Endesfelder D, Oestreicher U, Bucher M, Beinke C, Siebenwirth C, Ainsbury L, ….Goh VST, Chua CEL, Abend M & Port M. RENEB Inter-Laboratory Comparison 2021: The Dicentric Chromosome Assay. Radiation research 2023; 199(6): 556 -570.
Goh VST, Fujishima Y, Nakayama R, Takebayashi K, Yoshida M, Kasai K, Ariyoshi K & Miura, T. Manual Scoring with Shortened 48 h Cytokinesis-Block Micronucleus Assay Feasible for Triage in the Event of a Mass-Casualty Radiation Accident. Radiation research 2023; 199(4): 385-395.
Goh VST, Nakayama R, Blakely WF, Abe Y, Chua CEL, Chew ZH, Nakata A, Fujishima Y, Yoshida MA, Kasai K, Ariyoshi K, Miura T. Improved harvest and fixation methodology for isolated human peripheral blood mononuclear cells in cytokinesis-block micronucleus assay. International Journal of Radiation Biology 2021; 97(2): 194-207.

